Of the three particles that make up atoms (protons, neutrons, and electrons), which one do you think is the most important in the study of chemistry?
Well since chemistry is about chemical reactions, which means molecules decomposing or rearranging or combining with other molecules to make something new, how are those new connections made? The answer is through chemical bonds. And what are bonds made of? Electrons.
Understanding exactly how electrons are organized around the nucleus of an atom is going to be critical to understanding how chemical reactions work. For this reason, we are dedicating an entire module to the topic of the electronic structure of the atom.
That understanding begins with a fascinating phenomenon called atomic emission. When elements are subjected to the high temperatures in a flame or when an electrical current is passed through them, they emit beautiful and varied colors of light. It is atomic emission that produces the red light of a neon sign along with many of the colors you would see in a fireworks display.

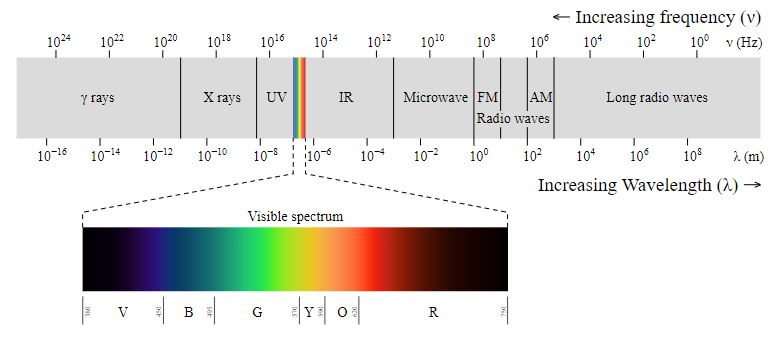
Light can be described as a wave. The wavelength, given the Greek symbol lambda, is the distance from peak to peak on a given wave. The wavelength would determine the color of the light wave. The amplitude is the distance from the midpoint to either the peak or the trough of a wave. The amplitude would affect the intensity of the light wave. The speed of the wave describes how fast it is moving through space. This is a constant for light in a vacuum but it varies when light passes through different materials. The frequency of a wave is the number of times that a peak passes by every second.

If the speed of a wave is held constant, the frequency will increase as the wavelength decreases. Waves with higher frequency have higher energies. Thus high energy corresponds to high frequency and short wavelengths. Likewise, low energy corresponds to low frequency and long wavelengths.
Visible light corresponds to wavelengths going from about 400 to 700 nm. Visible light is part of a much larger continuum of wave energy called the electromagnetic spectrum. On the high energy side of that spectrum, we would have gamma rays, followed by X-rays at a slightly lower energy, then ultraviolet light, and finally all the colors of visible light from violet to red. Moving beyond visible light toward the low energy side of the spectrum would be infrared light, microwaves, and ultimately radio waves.
How does this relate to atomic emission? Every element on the periodic table produces a unique atomic emission spectrum when it is exposed to a current or extreme temperatures. This is because there are electrons in each element, and those electrons exist at a particular energies. The lowest possible energy for a given electron is known as its ground state. When conditions are right for atomic emission, electrons in the ground state will jump to higher energy levels. However, just like anything else, what goes up must come down. For example, if I take a ball and throw it in the air, the natural process that happens is the ball falls back down. Well, excited electron does the same thing. It goes from an excited state back down to the ground state.
Now, because of the conservation of energy law, the energy that originally excited the electron to a higher energy can’t just be lost. Instead, the energy gets released as a little package of light called a photon.
So atomic emission occurs in three steps: Excitation, relaxation, emission of light. Heat or electrical energy that is absorbed during excitation gets released as light during relaxation.

Because different elements have different energy levels associated with their electrons, every element has a unique characteristic atomic emission spectrum. In other words, every element emits light at specific wavelengths, and those wavelengths combined together to give us the overall color that is produced. These wavelengths are actually a signature that can be used to identify elements. In fact, by observing atomic emission wavelengths is one way that astronomers can figure out which elements are present in distant stars.


Above are the unique atomic emission spectra of two elements, Calcium and Sodium. Can you spot the differences?